A Leading Global Medical
Research Institute
With one of the finest and advanced research laboratories in the region, the reliable findings from our research have enhanced our reputation as a leading global medical research institute.

Providing the Right Environment
for Mentorship and Training
We create an empowering environment for nurturing young minds to build the next generation of world class biomedical researchers in the African region.

Ultra-modern Laboratories
for Quality Research
Our ultra-modern laboratories enable research of the highest standard, leading to the development of new approaches in diagnosing, preventing, and controlling diseases of public concern.
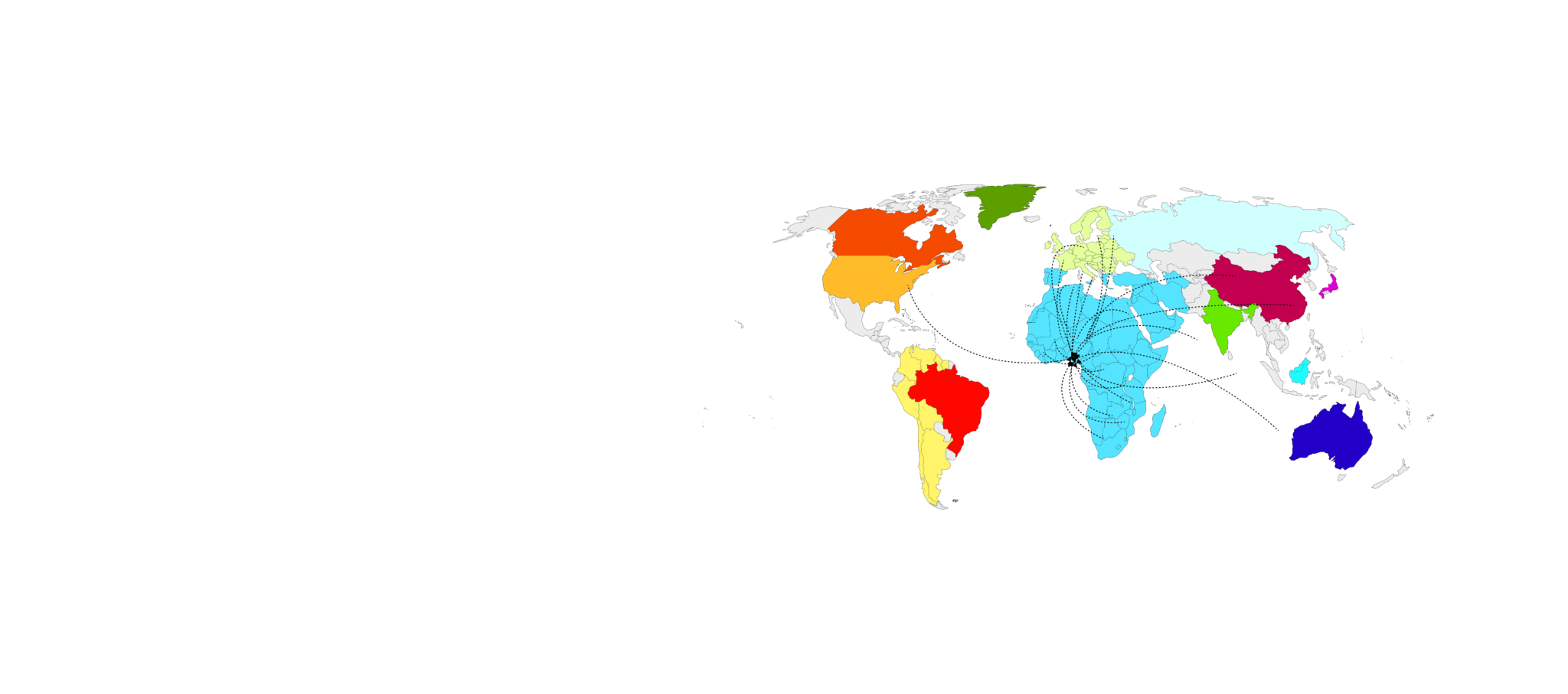
Our Global Reach
NMIMR’s broad and vibrant global network of collaborators and partners creates an avenue for fostering the development of intellect and resources.
Core Mandate
We conduct innovative research into communicable and non-communicable diseases
We provide high-end laboratory diagnostic and monitoring and surveillance services in support of global health
We provide capacity building in health services
News
World NTD's Day
Upcoming Events
Current Month
may
june
july
Our Networks
USA
Canada
Mali
Burkina Faso
Benin
Togo
Nigeria
Cameroon
Gabon
Congo
DR Congo
Mauritania
Egypt
Israel
Denmark
France
Spain
England
Germany
Sweden
Finland
Kenya
Uganda
Malawi
Zambia
Zimbabwe
Mozambique
Tanzania
South Africa
Ethiopia
Ghana
Ivory Coast
Senegal
Gambia
Cape Verde
Guinea
Sierra Leone
Liberia
Australia
Malaysia
China
Japan
India
Our Funders & Partners